National
Ebola Doctors Are Divided on IV Therapy in Africa

In this Oct. 27, 2014 file photo, health workers unload the lifeless body of a man suspected of contracting the Ebola virus, as they carry him to a grave site on the outskirts of Monrovia, Liberia. (AP Photo/Abbas Dulleh, File)
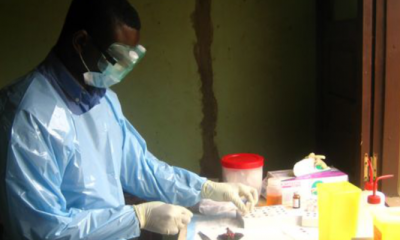
(New York Times) – Medical experts seeking to stem the Ebola epidemic are sharply divided over whether most patients in West Africa should, or can, be given intravenous hydration, a therapy that is standard in developed countries. Some argue that more aggressive treatment with IV fluids is medically possible and a moral obligation. But others counsel caution, saying that pushing too hard would put overworked doctors and nurses in danger and that the treatment, if given carelessly, could even kill patients.
The debate comes at a crucial time in the outbreak. New infections are flattening out in most places, better-equipped field hospitals are opening, and more trained professionals are arriving, opening up the possibility of saving many lives in Africa, rather than a few patients flown to intensive care units thousands of miles away.
The World Health Organization sees intravenous rehydration, along with constant measuring of blood chemistry, as the main reason that almost all Ebola patients treated in American and European hospitals have survived, while about 70 percent of those treated in West Africa have died.
Activism
Oakland Post: Week of April 17 – 23, 2024
The printed Weekly Edition of the Oakland Post: Week of April 17 – 23, 2024

To enlarge your view of this issue, use the slider, magnifying glass icon or full page icon in the lower right corner of the browser window. ![]()
Barbara Lee
Congresswoman Barbara Lee Issues Statement on Deaths of Humanitarian Aid Volunteers in Gaza
On April 2, a day after an Israeli airstrike erroneously killed seven employees of World Central Kitchen (WCK), a humanitarian organization delivering aid in the Gaza Strip, a statement was release by Rep. Barbara Lee (D-CA-12). “This is a devastating and avoidable tragedy. My prayers go to the families and loved ones of the selfless members of the World Central Kitchen team whose lives were lost,” said Lee.

By California Black Media
On April 2, a day after an Israeli airstrike erroneously killed seven employees of World Central Kitchen (WCK), a humanitarian organization delivering aid in the Gaza Strip, a statement was release by Rep. Barbara Lee (D-CA-12).
“This is a devastating and avoidable tragedy. My prayers go to the families and loved ones of the selfless members of the World Central Kitchen team whose lives were lost,” said Lee.
The same day, it was confirmed by the organization that the humanitarian aid volunteers were killed in a strike carried out by Israel Defense Forces (IDF). Prior to the incident, members of the team had been travelling in two armored vehicles marked with the WCF logo and they had been coordinating their movements with the IDF. The group had successfully delivered 10 tons of humanitarian food in a deconflicted zone when its convoy was struck.
“This is not only an attack against WCK. This is an attack on humanitarian organizations showing up in the direst situations where food is being used as a weapon of war. This is unforgivable,” said Erin Gore, chief executive officer of World Central Kitchen.
The seven victims included a U.S. citizen as well as others from Australia, Poland, the United Kingdom, Canada, and Palestine.
Lee has been a vocal advocate for a ceasefire in Gaza and has supported actions by President Joe Biden to airdrop humanitarian aid in the area.
“Far too many civilians have lost their lives as a result of Benjamin Netanyahu’s reprehensible military offensive. The U.S. must join with our allies and demand an immediate, permanent ceasefire – it’s long overdue,” Lee said.
Commentary
Commentary: Republican Votes Are Threatening American Democracy
In many ways, it was great that the Iowa Caucuses were on the same day as Martin Luther King Jr. Day. We needed to know the blunt truth. The takeaway message after the Iowa Caucuses where Donald Trump finished more than 30 points in front of Florida Gov. De Santis and former South Carolina Governor Nikki Haley boils down to this: Our democracy is threatened, for real.

By Emil Guillermo
In many ways, it was great that the Iowa Caucuses were on the same day as Martin Luther King Jr. Day.
We needed to know the blunt truth.
The takeaway message after the Iowa Caucuses where Donald Trump finished more than 30 points in front of Florida Gov. De Santis and former South Carolina Governor Nikki Haley boils down to this: Our democracy is threatened, for real.
And to save it will require all hands on deck.
It was strange for Iowans to caucus on MLK day. It had a self-cancelling effect. The day that honored America’s civil rights and anti-discrimination hero was negated by evening.
That’s when one of the least diverse states in the nation let the world know that white Americans absolutely love Donald Trump. No ifs, ands or buts.
No man is above the law? To the majority of his supporters, it seems Trump is.
It’s an anti-democracy loyalty that has spread like a political virus.
No matter what he does, Trump’s their guy. Trump received 51% of caucus-goers votes to beat Florida Gov. Ron DeSantis, who garnered 21.2%, and former South Carolina Gov. Nikki Haley, who got 19.1%.
The Asian flash in the pan Vivek Ramaswamy finished way behind and dropped out. Perhaps to get in the VP line. Don’t count on it.
According to CNN’s entrance polls, when caucus-goers were asked if they were a part of the “MAGA movement,” nearly half — 46% — said yes. More revealing: “Do you think Biden legitimately won in 2020?”
Only 29% said “yes.”
That means an overwhelming 66% said “no,” thus showing the deep roots in Iowa of the “Big Lie,” the belief in a falsehood that Trump was a victim of election theft.
Even more revealing and posing a direct threat to our democracy was the question of whether Trump was fit for the presidency, even if convicted of a crime.
Sixty-five percent said “yes.”
Who says that about anyone of color indicted on 91 criminal felony counts?
Would a BIPOC executive found liable for business fraud in civil court be given a pass?
How about a BIPOC person found liable for sexual assault?
Iowans have debased the phrase, “no man is above the law.” It’s a mindset that would vote in an American dictatorship.
Compare Iowa with voters in Asia last weekend. Taiwan rejected threats from authoritarian Beijing and elected pro-democracy Taiwanese vice president Lai Ching-te as its new president.
Meanwhile, in our country, which supposedly knows a thing or two about democracy, the Iowa caucuses show how Americans feel about authoritarianism.
Some Americans actually like it even more than the Constitution allows.
About the Author
Emil Guillermo is a journalist and commentator. He does a mini-talk show on YouTube.com/@emilamok1.
-

 Activism4 weeks ago
Activism4 weeks agoOakland Post: Week of March 27 – April 2, 2024
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoCOMMENTARY: D.C. Crime Bill Fails to Address Root Causes of Violence and Incarceration
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoFrom Raids to Revelations: The Dark Turn in Sean ‘Diddy’ Combs’ Saga
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoCOMMENTARY: Lady Day and The Lights!
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoMayor, City Council President React to May 31 Closing of Birmingham-Southern College
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoBaltimore Key Bridge Catastrophe: A City’s Heartbreak and a Nation’s Alarm
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoBaltimore’s Key Bridge Struck by Ship, Collapses into Water
-

 #NNPA BlackPress4 weeks ago
#NNPA BlackPress4 weeks agoBeloved Actor and Activist Louis Cameron Gossett Jr. Dies at 87